Mercolized Wax
For centuries creams and ointments containing mercury compounds were used to treat a wide variety of skin complaints including syphilis, impetigo, acne and warts. Dermatologists of the nineteenth century also employed it as a lightening agent to treat pigmentation problems such as ‘liver spots’, so it comes as no surprise that many nineteenth-century cosmetics and patent medicines also contained forms of mercury. For example, Harriet Hubbard Ayer’s Recamier Cream’s ‘beneficial’ effects on the complexion were due to mercury (Albert, 2000, p. 522).
See also: Recamier Manufacturing Company.
Despite numerous warnings of their dangers, a number of cosmetics, such as freckle removers, relied on them to “improve and beautify the complexion” and the use of mercury compounds such as bichloride of mercury (corrosive sublimate) and ammoniated mercury continued well into the twentieth century.
Freckle Milk (Lait Antéphelique). Camphor 1¾ oz. Ammonium chloride ¾ oz. Corrosive sublimate 150 grains Albumen 3½ oz. Rose water 2 lb. We call attention to the fact that the sublimate (bichloride of mercury) is very poisonous and must be used with the greatest care.
(Askinson, 1923, p. 302)
See also Freckle Removers.
Dearborn Mercolized Wax
In sunny countries like Australia, face creams that helped remove pigmentation defects and lighten the skin were very popular. One of the more common products of this type was Dearborn’s Mercolized Wax. The name ‘Mercolized Wax’, was not a chemical term but rather a trademark taken out by the Dearborn Supply Company in 1911.
Dearborn made and distributed Mercolized Wax in a number of countries during the first half on the twentieth century and advertised its benefits and uses accordingly. It was suggested that the wax should be applied at night and washed off in the morning and/or in the morning in a light film as a powder base.
Instead of greasy face creams use Mercolized Wax and watch the rapid and permanent improvement in your complexion. Creams mask blemishes; Mercolized Wax removes them. Mercolized Wax stimulates the pores to natural action, absorbs and takes away every particle of waste matter. It leaves the skin exquisitely smooth and fine, reveals its natural beauty. Every night, every morning, use Mercolized Wax this way.
First-wash
Night and morning, cleanse the skin with warm, not hot, water, and a good soap. Pilenta is best because it is free from excess alkali. It soothes; comforts.
Then pat it
Use a soft huckaback towel, not to rub the skin this way and that, stretching it in all directions, but to pat it until superfluous moisture is removed.
Now-apply
Now apply Mercolized Wax. Do not merely smear the face with it. With fingertips pat the Wax smoothly and evenly over the face, neck, and beneath the chin.
Massage
Finally, massage gently, using circular upward and outward movements. This Wonderful Wax will absorb and thus remove impurities, which, if neglected, choke the pores.
Mercolized Wax is guaranteed not to contain any form of mercury and does not encourage hair growth.(Dearborn advertisement, 1925)
Although the product was advertised as “guaranteed not to contain any form of mercury and does not encourage hair growth” it did contain mercury. Mercolised Wax was analysed by the Kansas State Board of Health and some years later by the New Haven Department of Health. They both found that it contained:
Ammoniated mercury 10 per cent.
Zinc oxide 10 per cent.
Ointment base (petrolatum and paraffin).
These reports put the mercury content of the product on the high side, so, it is little wonder that the American Medical Association (AMA) in 1912 reported that the “stuff is a caustic poison and in the interest of the public safety the law should require that it be labeled as such” (AMA, 1912, p. 603).
Use of mercury compounds
Some constraints were placed on mercury compounds in the late 1930s. In 1939, the American Food and Drug Administration (FDA) declared that they considered ammoniated mercury to be a drug but allowed products to contain 5% or less of ammoniated mercury if they included conspicuous warnings on the packaging stating that if skin irritation appeared the use of the product should be discontinued.
In 1939, U.S. Federal Trade Commission (FTC) ordered the Dearborn Supply Company to stop representing that Mercolized Wax absorbs surface skin, surface discolorations, or removes coarseness, blackheads, freckles or sunburn or softens the skin and reveal that Mercolized Wax contains ammoniated mercury. Failure to do this led the FTC to take the company to court in 1944 because its advertisements failed to “reveal the harmful consequences that may result from indiscriminate use of ‘Mercolized Wax,’ a cosmetic preparation containing ammoniated mercury” (Kesaris, 1985). Presumably the delay between the warning and the court action was in part a result of the outbreak of the Second World War.
These minor constraints meant that skin-lightening cosmetics containing mercury compounds continued to be made in the late twentieth century despite the known dangers. Writing in Soaps, Perfumes & Cosmetics, A. J. Lehman (a representative of the FDA) noted that:
Mercury compounds have been used in cosmetics since antiquity. However, they cannot be regarded as innocuous. Actually mercury and its compounds are extremely toxic. There is little or no margin of safety between effective and toxic levels. Sensitization to mercury compounds is not uncommon.
The principle application of mercury is in the form of ammoniated mercury used in bleaching creams. The action of mercury is to arrest DOPA, an enzyme, dihydroxy-phenyl analine, which is essential in the process of conversion of tyrosine to melamine in the skin. We have regarded such articles in the category of drugs for self-medication that should conform to the following restrictions:
1. Ammoniated mercury preparations should not exceed 5 per cent concentration.
2. Mercuric bichloride and other mercurials must not exceed 0.2 per cent concentration.
3. “Use” to be immediately discontinued if irritation develops.
4. Not to be used on damaged skin, such as shaving “nicks” or after a depilatory.
5. There should be a preliminary test for sensitization to mercury.
6. Preparations are not to be used on children below twelve years of age.(Lehman, 1960, p. 396)
As late as 1972, Balsam and Sagarin described ammoniated mercury as a poison when ingested and noted that it needed to be treated with care but still included a number of skin-lightening formulae that used it.
Part A Carolate 15.00% Ceraphyl 28 31.00% Ceraphyl 140A 20.00% Cosmetic liquid 585 10.00% Ozokertite 95° 5.00% Beeswax 5.00% Ceraphyl 424 5.00% Butyl paraben 0.20% Part B Zinc oxide 5.00% Bismuth subnitrate 0.25% Ammoniated mercury 3.00% Perfume 0.55% Procedure: Melt the components of Part A together, heating to 126 to 128°F. Do not exceed a temperature of 130°F. With stirring, add B. Cool to 110°F, and add perfume. Pass the mixture through a colloid mill into a stainless steel holding tank.
(Sagarin & Balsam, 1972, p. 232)
Effects of mercury on skin pigmentation
By the 1970s, the action of mercury compounds on skin pigmentation had been ascertained. In addition to reducing melanin formation by inhibiting the enzyme tyrosinase, some mercury salts, including mercuric chloride and ammoniated mercury, were also shown to have an exfoliating effect on the epidermis due to the generation of hydrochloric acid when the mercury salts reacted with the skin. If enough acid was produced to generate this ‘sloughing effect’, the exfoliation would add to the lightening process.
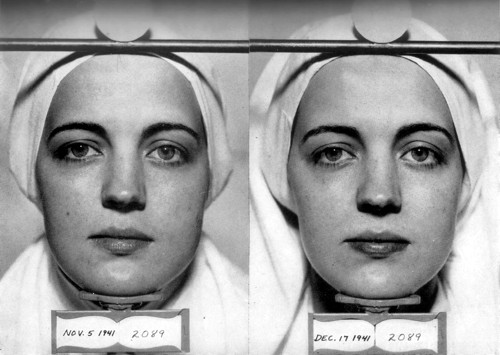
Lightening of the skin produced by ammoniated mercury. Left: Before treatment. Right: After 6 weeks of using ammoniated mercury. Harry does not indicate the concentration of ammoniated mercury that was used. (Harry, 1955, p. 32)
Legislation
During the 1970s, restrictions or prohibitions were placed on the use of mercury compounds in the UK, the USA , the EU and elsewhere. By the 1980s, Harry’s Cosmeticology noted that:
In a modern context, ammoniated mercury can scarcely be considered to be a suitable material for use in a cosmetic product which will be applied regularly, day by day, despite its existence in pharmacopoeial formulae.
(Wilkinson & Moore, 1982, p. 267)
However, despite the fact that using mercury in cosmetics is now prohibited in the United States, Europe, Australasia and elsewhere, cosmetics containing mercury compounds still make regular appearances in the reports of public health agencies in these countries. Skin lightening products are still made and used in Africa, South America and Asia and these products are mail-ordered or carried in luggage into the developed world despite import prohibitions. Low income groups with pigmented skins are particularly susceptible to using these banned skin-lightening cosmetics. They are potentially very serious as they are often applied over the whole body and not just the face, which increases the amount of mercury that gets absorbed.
Updated: 17th December 2018
Sources
Albert, M. R. (2000). Nineteenth-century patent medicines for the skin and hair. Journal of the American Academy of Dermatology, 43, 519-526.
American Medical Association (1912). Nostrums and quackery (2nd ed.). Chicago: American Medical Associated Press.
Askinson, G. W. (1923). Perfumes and cosmetics: Their preparation and manufacture (5th ed.). London: Crosby Lockwood and Son.
Harry, R. G. (1955). Modern cosmeticology. (4th ed.). London: Leonard Hill.
Kesaris, P. (Ed.). (1985). Records of the Federal Trade Commission. University Bethesda: Publications of America.
Lehman, A. J. (1960). Drugs in cosmetics: Should they mix? Soaps, Perfumes and Cosmetics. April.
Sagarin, E., & Balsam, M. S. (Eds.). (1972). Cosmetics: Science and technology (2nd ed.). New York: Wiley-Interscience.
Wilkinson J. B., & Moore, R. J. (Eds.). (1982). Harry’s cosmetology (7th ed.). New York: Chemical Publishing.

1919 Mercolized Wax. The Dearborn Company is not identified in this advertisement but they had taken out a trademark for the name Mercolized Wax in 1911.

1926 Dearborn Mercolized Wax.

1928 Othine Double Strength. Ammoniated mercury was found in many commercial freckle removers.

1931 Dearborn Mercolized Wax.

1933 Dearborn Mercolized Wax.

1935 Dearborn Mercolized Wax.

1952 Dearborn Mercolized Wax Cream.

1956 Dearborn Mercolized Wax.

Tin of Dearborn Mercolized Wax sold in Australia probably from the 1950s-60s. The back of the tin reads: “First, cleanse the skin with warm not hot water. Next, use a soft huckaback towel; do not wipe, but pat the skin gently to remove superfluous moisture. Now apply Mercolized Wax. Do not merely smear the face with it. With fingertips pat Wax smoothly and evenly over the face, neck and beneath the chin. Finally, massage gently, using circular upward and outward movements and leave on all night. In the morning wash off with warm water, and you will soon have a vastly improved complexion.”
Unfortunately, the petroleum base gives Dearborn’s Mercolized Wax a long shelf life. Should you find an old jar or tin, make sure you wash your hands after touching the cream to avoid getting mercury into your body through your mouth.

Recent jar of Dearborn Night Cream from England. The back of the container reads:
“The emolients in which this Night Cream is based will help to keep your skin soft and supple and gently discourage lines and dryness.
Nightly Beauty Routine
After cleansing, massage the cream gently into the skin using upward strokes and light fingertip movements over facial contours.”

La Dainty Bleaching Ointment. Dearborn was not the only company to make bleaching cosmetics containing ammoniated mercury.
