Hirestra Laboratories (Endocreme)
Hirestra Laboratories, Inc. was founded at 745 Fifth Avenue, New York in 1936 by Robert I. Rheinstrom [1882-1959]. In 1937, the company introduced Endocreme, a hormone cream containing 10,000 International Units (IU) per ounce of estradiol (dihydroxyestrin), a natural hormone produced by the Schering Corporation of Bloomfield, New Jersey.

Above: 1937 Endocreme and instructions booklet.
Schering had spent two years examining the safety and efficacy of estradiol but their tests would not be considered rigorous. These started with the private patients of Dr. Max A. Goldzieher, moved on to the patients of a number of endocrinologists and dermatologists, and then finished with 30 patients at the Grassland’s Hospital, Valhalla, New York. All indications were that estradiol was both efficacious and safe (‘Hearings before the House Select Committee,’ 1951, pp. 1124-1125).
American Medical Association
In September, 1937, as Endocreme was being introduced to the general public, Rheinstrom and a colleague flew to Chicago to inform Dr. Morris Fishbein [1889-1976], the editor of the Journal of the American Medical Association (JAMA), about the product. Rheinstrom received assurances from Dr. Fishbein, but JAMA subsequently published a scathing attack on Endocreme in April, 1938, describing it as a menace.
For more than year this product, manufactured by the Hirestra Laboratones, Inc, of New York, has been offered to the public without a word of warning that it may be a potential menace to health and life. Now, on the basis of available evidence, which the manufacturer should have had before the product was sold openly to the pubic, THE JOURNAL is warranted in warning users of this product that its indiscriminate use may lead to dangerous consequences, that it possesses the potentiality of bringing about serious changes in the genital and reproductive organs of women, that it may induce changes in the breasts, and that there is, in addition the possible disorganization of the menstrual cycle, the potentiality of the production of cancer.
(‘Endocreme—a cosmetic with a menace,’ 1938, p. 1194)
Rheinstrom considered this to be libellous and filed suit against the AMA in 1938 . However, he dropped the case in 1940 after receiving money from Schering. The AMA was refusing to give Schering a seal of approval as long as the case continued. (‘Hearings before the House Select Committee,’ 1951, p. 1138) and Schering was prepared to pay Rheinstrom to drop the case in exchange for the approval. The AMA did not pay compensation and their opposition to the use of hormones in cosmetics continued unabated.
In 1938, while the court case was still running, the Food, Drug and Cosmetic Act (FD&CA) passed in the United States. Fortunately for Hirestra, the Food and Drug Administration (FDA), which was charged with administering the Act, did not ask for Endocreme to be withdrawn from the market. They later placed limits on the amount of hormone allowed in cosmetics but Endocreme was already complying with that stipulation so it was unaffected.
See also: Hormone Creams, Oils and Serums
Products
Hirestra’s Endocreme was meant to be used as a night cream. This would allow it to remain on the skin for at least six hours so that the estradiol could be absorbed.
Endocreme Face Cream: “Far more than a luxurious night cream, Endocreme’s natural female hormones are absorbed by the skin, work beneath the surface to help offset the usual causes of ageing and dryness.”
In addition to the face, Hirestra also suggested it for the neck, and hands and the company provided detailed instructions on how to achieve the best results.
1. Endocreme is a night cream, to be applied before retiring, and allowed to remain on the skin for six hours, so the Dihydroxyestrin (now more generally called Estradiol) may be fully absorbed. The use of Estradiol in a beauty cream is exclusive to Endocreme.
2. The nightly dose, for face, neck and hands, is 2 grams (about ½ teaspoonful measured flat). But you need not measure. If you are using Endocreme only for your face, neck and hands, and if your jar is used up in from 30 to 35 nights, the amount is correct.
3. When applying Endocreme, be sure that the skin is thoroughly clean and dry. Use mild soap and soft water—or use your regular cleansing cream—but be sure to wipe the skin dry with cleansing tissues before you apply Endocreme. Otherwise, the Endocreme will be diluted and may work more slowly.
4. Now, with your finger tips massage Endocreme gently but very thoroughly into your skin, from the center of the forehead outward to the temples. Continue this gentle but thorough massage until you have rubbed your Endocreme into the skin of every area you wish to treat. Always massage in the direction shown by the arrows in the Endocreme “Venus Diagram”.
5. On the backs of your hands stroke Endocreme upward from your fingers to your wrists, or higher. Then pat the underchin and throat with the backs of your hands.
6. The time spent in applying Endocreme is well spent, because the special ingredient is absorbed through the hair follicles into the sweat glands or through the sebaceous glands, and from there into the surrounding tissues. Endocreme cannot do its work until it has been absorbed.
7. Be sure all facial massage is gentle. Harsh massage is unwise.
8. Use Endocreme at night, every night, and leave it on all night for full absorption; it will not stain pillows or nightclothes. It is prepared from fine oils in a combination which melts readily at body temperature. If the contents of the jar show a tendency to melt, keep in a cooler place.(Endocreme advertisement, 1937)
Also see the sales booklet: Answers to questions your customers will ask about Endocreme (1937)
Subsequent to its introduction, Hirestra introduced to additional products: Endocreme Cleansing Cream (1941) which was hormone-free; and Endocreme Hand Lotion (1945) containing Activol. Hirestra began using the term Activol in preference to estradiol in 1940. Hirestra described it as “ a scientific replacement for woman’s own natural skin-vitalizing substance”. Why Hirestra began doing this is unknown to me. They did not trademark the name but, given that its use started in the same year as the settlement of the court case, it may have been part of the agreement Rheinstrom reached with Schering.
Endocreme Cleansing Cream: “[T]he ideal akin cleanser that helps hasten the results of ENDOCREME Face Cream.”
Endocreme Hand Lotion: “The only ‘Beauty Treatment’ for the hands. It alone contains ACTIVOL (natural selected hormones) in a special suspension beneficial to hand skin.”
The addition of Endocreme Hand Lotion meant that the original Endocreme was now often referred to as Endocreme Face Cream.
Sale
In 1946, Robert Rheinstrom sold Hirestra Laboratories but I have no definitive records of who became the new owners. William Erhardt Weiss Jr. [1913-1885] was made the new president of Hirestra Laboratories in June, 1946, the same month be bought Kathleen Mary Quinlan and both Quinlan and Hirestra became subsidiaries of Natcon Industries, Inc. also founded in 1946.

Above: 1966 Endocreme by Jacqueline Cochran, part of a post christmas sale advertisement by McCurdy’s.
However, Hirestra Laboratories, Inc. also became a division within Jacqueline Cochran. Cochran sold Endocreme products through her outlets after 1946 as did Shulton following their acquisition of Jacqueline Cochran in 1965. This raises the question of who owned Hirestra. The only solution I have to this conundrum is that perhaps Jacqueline Cochran bought the distribution rights to Endocreme while manufacturing remained with Natcon.
The new owners of Endocreme started with some minor changes. The Endocreme Face Cream jar underwent some small labelling modifications and the company began giving away every sixth jar free. Greater emphasis was also placed on the fact that Endocreme Face Cream and Endocreme Hand Lotion (Hand Beauty) contained hormones.
Endocreme Hand Beauty: “Contains natural hormones that benefit the skin. Just a very little lotion, in fact an amount the size of a dime, is required for one application. It is absorbed quickly and is not oily or sticky. Incidentally, this Hand Beauty lotion makes an excellent powder base, too.”
Other additions to Endocreme included Endocreme For Younger Women (1949) also known as Endocreme Light; Endocreme Hormone Oil (1952); Endocreme Liquid (1954); and Endocreme Entrance (1955), another cleanser.

Above: 1949 Endocreme Regular in a black jar and Endocreme For Younger Women (Light) in a white jar.
Endocreme For Younger Women: “In ‘designing’ a hormone cream for younger skins that aren’t hungry for rich, heavy oils, we’ve retained exactly the same hormone potency that you find in regular Endocreme, yet the cream is quickly absorbing and very light in texture. It’s a ‘natural’ for summer.”
Endocreme Hormone Oil: “[R]eplenishes the skin’s natural waning hormone supply, tends to overcome skin dryness, tiny wrinkles, by restoring normal moisture deep within the skin . . . makes the skin firmer by increasing circulation in tiny, collapsed blood vessels. No non-hormone cosmetic can aid in recapturing skin beauty this way.”
Endocreme Liquid: “[D]esigned to help moisten and smooth your skin. Its wonder formula includes Endo-Activol, a selected natural hormone, plus lipoids and lanolin.”
Endocreme Entrance: “[W]elcomes the benefits of beauty treatments to the innermost cell depths removes every obstacle to youthful skin beauty! The pores of your skin act as tiny ‘traps’ for make-up, soot, bacteria so your beauty treatment must gain entrance to the vital tissue under the skin surface.”

Above: 1956 From left to right: Endocreme Cleansing Cream, Endocreme Entrance, Endocreme Face Cream, Endocreme Liquid, and Endocreme Hand Lotion.
Hormones were also used in Endocreme Hormone Hair Beauty (1949), an anti-dandruff and falling hair treatment applied like a brilliantine which was combined with a Shampoo and Conditioner into a treatment group.
Endocreme Hormone Hair Beauty: “[U]ses hormones to attack hair troubles at the root, and offers a definite home beauty program complete with SHAMPOO and SCALP CONDITIONER – 4 OUT OF 5 women tested by doctors definitely cleared up dandruff, itchy scalp, falling hair, those three robbers of natural hair beauty!”
The only other products developed for Endocreme included Instant Endocreme (1957) and Endocreme Moisturizing Cleanser (1958). Neither product contained hormones.
Instant Endocreme: “[A] light, quick absorbing cream . . . gives your skin a miraculously fresh look . . . contains ingredients which have the power to rekindle the true, glowing, ‘aliveness’ of youth.”
Endocreme Moisturizing Cleanser: “[H]as amazing deeply penetrating cleanser ability . . . not only cleanses most effectively but also nourishes. Leaves skin marvellously clean and fresh, fully, ‘moisturized’ and supple, ready for make-up or night treatment. Specially beneficial for dry skin.”
Like earlier Endocreme cleansers, the Moisturising Cleanser was to be used to hasten the penetration of Endocreme but as well as removing grit, dust and make-up, it moisturised as it cleansed.
Instant Endocreme in jars or tubes contained Neo-Activol, Alphatrocopherol Acetate, Calciferol, and Vitamin D (Stabile, 1969, p. 90). It was recommended for use during the day under powder or make-up after the skin was cleansed with Moisturising Cleanser but it could also be applied at night.
How to use Instant Endocreme:
1. Cleanse skin thoroughly with Endocreme Moisturizing Cleanser. Remove cleanser thoroughly.
2. Pat Instant Endocreme into face, neck and throat with upward and outward strokes. Wait five minutes, gently massage the protective film that remains. No trace or cream remains on the skin—you’ll go to bed with a glow of good looks.(Hirestra pamphlet for Endocreme, c.1957)
Some changes were also made to the original Endocreme. In 1959, the product was buffered to give it a pH closer to that of the skin. This, it was claimed, resulted in “faster absorption and quicker, more effective results”.

Above: Buffered Endocreme from Jacqueline Cochran (Smithsonian).
See also: Acid Creams
Later developments
In 1965, Jacqueline Cochran was bought by Shulton. Endocreme and Endocreme Liquid continued to be sold by Shulton and were still on the market after Shulton was sold to American Cyanamid in 1971. However, like other over-the-counter (OTC) products containing hormones they were discontinued in the 1970s.
Also see: Jacqueline Cochran and Shulton
Timeline
| 1936 | Hirestra Laboratories, Inc. founded in New York. |
| 1937 | Hirestra Laboratories moves to 551 Fifth Avenue. New Products: Endocreme. |
| 1939 | New Products: Endocreme Cleansing Cream. |
| 1945 | New Products: Endocreme Hand Lotion. |
| 1946 | Endocreme bought by Jacqueline Cochran. Hirestra Laboratories moves to 487 Park Avenue. |
| 1948 | New Products: Endocreme Hand Beauty. |
| 1949 | New Products: Endocreme Light; and Endocreme Hormone Hair Beauty, Shampoo and Conditioner. |
| 1952 | New Products: Endocreme Hormone Oil. |
| 1954 | New Products: Endocreme Liquid. |
| 1955 | New Products: Endocreme Entrance. |
| 1957 | New Products: Instant Endocreme. |
| 1958 | New Products: Endocreme Moisturizing Cleanser. |
| 1965 | Jacqueline Cochran acquired by Shulton. |
| 1971 | Shulton bought by American Cyanamid. |
First Posted: 20th October 2020
Last Update: 25th May 2022
Sources
Endocreme—a cosmetic with a menace. (1938). The Journal of the American Medical Association, April 9, 1194-1196.
Hearings before the House Select Committee to investigate the use of chemicals in foods and cosmetics, House of Representatives, eight-second congress, first session. (1951). Part 1, 1123-1147.
Stabile, T. (1969). Cosmetics: Trick or treat? (3rd ed.). New York: Arco Publishing Company, Inc.

1937 Endocreme.
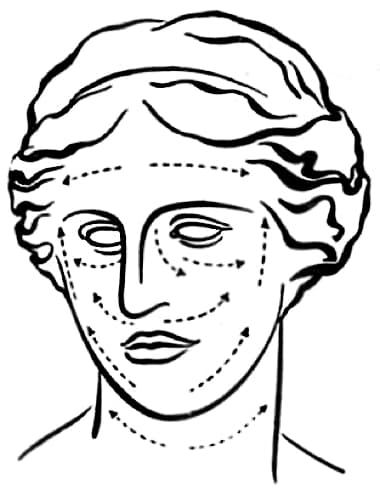
1937 The ‘Venus Diagram’ showing the massage movements to be used when applying Endocreme.

1938 Endocreme.

1940 Endocreme.

1941 Endocreme.

1944 Endocreme.

1946 Endocreme.

1948 Endocreme Face Cream.

1951 Endocreme.

1955 Endocreme Liquid with graduations marked on the side of the container to allow the product to be measured out.

1957 Instant Endocreme.

1957 Instant Endocreme in a tube.

1966 Jacqueline Cochran products now owned by Shulton. Endocreme Liquid and Endocreme (right). Endocreme Liquid is now in a black bottle.
