Desincrustation
A galvanic treatment often combined with iontophoresis is ‘de-incrustation’. This treatment – developed in the early 1930s by the Société P.A.B. in France – used a direct current to remove ‘incrustations’ from the skin.
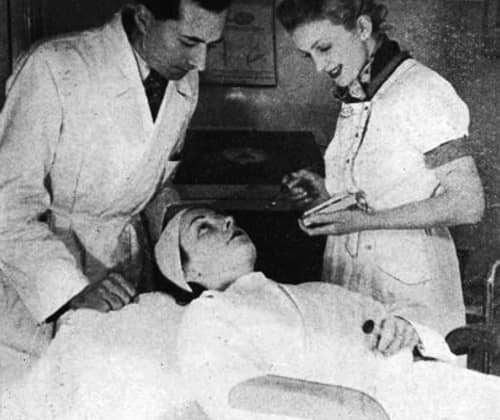
Above: 1937 René Guinot supervising a desincrustation treatment.
See also: Iontophoresis
The developers of the treatment described these ‘incrustations’ as ‘microscopic crystallisations’ formed by chemical reactions between certain chemicals and minerals in creams, make-up, atmospheric pollution and perspiration. As the impurities built up they clogged the skin causing wrinkles, pimples, acne and blackheads. They had to be removed, that is, the skin had to be ‘de-incrustated’.
The chief characteristics of the method consist in breaking up, reducing and eliminating all the impurities (waste matter, dust, toxins, crystallisations, etc.) which block up the glandular tubes. … The immediate result is—increased blood circulation and, gradually, recolouring of the epidermis.
(The Hairdresser and Beauty Trade, 1934)
Incrustations could be eliminated without electricity either by undertaking a deep-cleansing salon facial treatment – using cleansers, warm sprays and massage – or by using a facial scrub at home. In France these treatments were also known as désincrustation but in the English speaking world the term usually only applies to the electrical treatment.
Basic principles
Electrical desincrustation relies on a well-known effect of direct (galvanic) currents, that an alkali (sodium hydroxide) is produced at the negative electrode and an acid (hydrochloric acid) is generated at the positive electrode. This chemical reaction was well known in the nineteenth century and was the basic science behind the electrical removal of unwanted hair through electrolysis.
See also: Electrolysis
Unlike electrolysis, where the sodium hydroxide (lye) is concentrated in a small area in the living dermis of the skin through the insertion of a needle, in desincrustation the sodium hydroxide is spread over the stratum corneum of the epidermis. So, rather than destroying tissue, as in electrolysis, in desincrustation the sodium hydroxide merely softens the keratin in the epidermis. This assists in desquamating surface keratinocytes and helps loosen any hard plugs of sebum including blackheads.
Using sodium hydroxide created by a galvanic current to cleanse the skin surface was not a new idea. It had had been previously employed in the Electrolytic Cup, an American device that combined vacuum suction with an electric current to loosen and remove dirt and other skin debris.
See also: Electrolytic Cup
Like iontophoresis, the negative electrode used in desincrustation can be a disk, roller or ball electrode, a full facial mask, or something as simple as a tweezer electrode encased in a pad of cotton wool soaked in conducting solution. Unlike iontophoresis, where specialised ampoules are needed, a simple salt solution is all that is required. One can be made up using the following formula:
| Baking soda (sodium bicarbonate) | 5 ml (1 teaspoon) |
| Distilled water | 250 ml (1 cup) |
Sodium bicarbonate beaks down into sodium (positive) and bicarbonate (negative) ions when dissolved in water. This creates a slightly alkaline solution which is more effective than using common table salt (sodium chloride). In the 1930s, I believe a copper sulphate solution was also used but this would not be recommended today.
The suggested treatment in the 1930s started with a cleansing massage to soften and hydrate the skin as much as possible. A warm pulveriser or steam bath under coloured light may also have been used to improve the skin hydration.
See also: Vaporisers (Steamers & Atomisers)
The client was then connected to the positive electrode while the operator worked on the face with the negative electrode saturated with the desincrustation solution. The session generally lasted about 10 to 15 minutes depending on the strength of the electric current and the sensitivity of the skin to electricity. The strength of the current was adjusted to suit the client with most subjects withstanding a current of between ½ to 3 milliamps.
Combining treatments
In order to carry out either desincrustation or iontophoresis, a salon had to purchase a galvanic machine. Having done so it made sense for them to maximise the return on their outlay by combining iontophoresis and desincrustation into a single treatment. This was commonly done by first carrying out desincrustation, using the negative electrode, and then following this with iontophoresis, using the positive electrode.
The reasoning behind this is as follows. Starting with desincrustation reduces the barrier properties of the skin by assisting with exfoliation, in theory making it easier for ions to move across the skin during iontophoresis. Then, as acid is produced under the positive electrode during iontophoresis, this helps to restore the acid balance of the skin upset by the alkali generated during desincrustation.
Updated: 31st August 2018
Sources
Cressy, S. (2004). Beauty therapy fact file (4th ed.). Oxford: Heinemann Educational Publishers.
Désincustation electrique. (1937). La Parfumerie Moderne. June, Number 6, 235-239.
Gallant, A. (1980). Principles and techniques for the beauty specialist (2nd ed.). Cheltenham: Stanley Thornes.
Guinot, R. (1937). La peau et la désincustation. La Parfumerie Moderne. November, Number 11, 417-223.
The hairdresser and beauty trade. (1934). London.
Lloyd, E. (1907). The skin. Its care and treatment (3rd. ed.). Chicago: McIntosh Battery and Optical Company.
Lloyd, E. (1914). The skin. Its care and treatment (5th ed.). Chicago: McIntosh Battery and Optical Company.
McIntosh Battery and Optical Company. (1903). The skin. Its care and treatment. Chicago: Author.
Simmons, J. V. (1989). Science and the beauty business. Volume 2. The beauty salon and its equipment. London: Macmillan.
Wall, F. E. (1946). The principles and practice of beauty culture (2nd ed.). New York: Keystone Publications.

1934 Société P.A.B. (Produits et Appareils de Beauté ) advertising their desincrustation treatment. The company was founded in 1930.

1934 A French salon advertising epilation treatments and desincrustation using the PAB method.

1935 Société P.A.B at 5 Rue Tronchet, Paris.

1937 Desincrustation apparatus as used by P.A.B. (Produits et Appareils de Beauté).
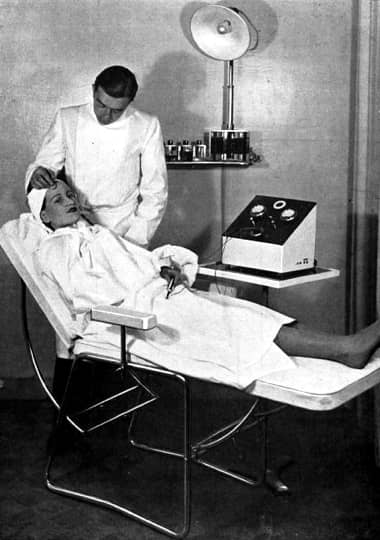
1937 Client undergoing electrical desincrustation.
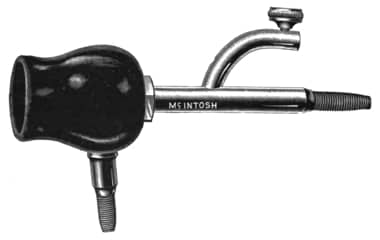
McIntosh Electrolytic Cup. This earlier American cleansing device also used a galvanic current to generate an alkali but combined it with vacuum suction.
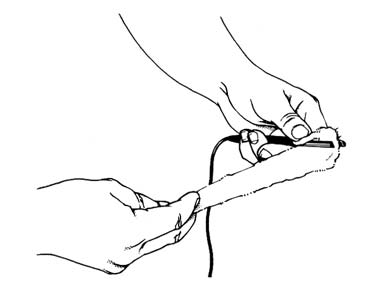
For desincrustation, cotton wool can be wrapped around a tweezer electrode and then dipped in salt solution (Gallant, 1980).
