Liquid Face Powders
Despite the fact that ‘powder and paint’ was still frowned on at the end of the nineteenth century, the use of liquid powders was not uncommon. Made by suspending powder in water, these cosmetics – sometimes called evening whites – were applied with a sponge to the neck, décolletage, shoulders, back, arms and hands to whiten and even the tone of skin that was not covered by an evening dress.
How Ladies Learn to “Make Up”
A celebrated theatrical costumier told our interviewer that he was frequently asked to coach ladies in private life in the art of “making up“ for the drawing room and the promenade, the usual aim being the acquisition of bright eyes and delicate skins. “There is,” said he, “a great run on kohl for the eyes, and, besides belladonna, I am often asked for primadonna—a similar article. Some women wear wash-leather masks during sleep to keep the skin of their faces soft, and a great many of them use a sort of enamel—sold liquid in bottles and applied with a piece of sponge—for their shoulders and arms in evening dress. Of course, theatrical grease paints cannot be used in daylight; they are too crude.(Forget-Me-Not, 1893, p. 85)

Above: 1886 Dorin Blanc Daniel à la Glycérine and Créme Daniel à la Glycérine. The clear supernatant can be seen at the top of the Blanc Daniel bottle. The Crème Daniel is probably more like a lotion than a cream.
The use of liquid powders continued into and through the twentieth century, eventually leading to the development of the liquid foundations that became popular after the Second World War.
Early liquid powders
There were three types of liquid powder in use at the end of the nineteenth century that played a part in the development of this cosmetic in the twentieth century: calamine lotion, made by the pharmacy trade; a theatrical make-up known as wet white; and a group of cosmetics that can be referred to as liquid pearls. All these preparations were made from powders suspended in water and anyone making them had to deal with similar technical problems; that is how to: keep the powder suspended; get it to stick to the skin; make it tone with the skin; and get it to go on without streaking.
Also see the company booklet: Beauty’s Boudoir (1886)
Calamine Lotion
True calamine, as mentioned in the British Pharmacopeia of 1887, is native zinc carbonate, a brownish to pinkish powder. By 1900, interest in native calamines had waned – mainly due to native deposits being worked out in many countries – and since then products labeled calamine have been made from mixtures of zinc carbonate and zinc oxide, tinted pink with iron oxides such as Armenian bole.

Above: Calamine lotion. It is stored in a brown bottle as zinc oxide is affected by light which will turn the mixture grey over time. The mucilaginous base suggests the suspension is being stabilised with gums.
Calamine lotion is used as an anti-pruritic to reduce itching caused by sunburn, insect bites and minor rashes. Zinc oxide is a mild antiseptic and an astringent so it helps the healing process. Liquid phenol (carbolic acid) is frequently added to modern versions as an additional disinfectant and topical anaesthetic. All formulations, past and present, generally included glycerine which acted as a moisturising agent, improved the suspension, and helped the powder stick to the skin.
As the mixture is a suspension, instructions to shake the lotion vigorously before using are generally included on the label. The stability of the suspension is improved by making the powder as fine as possible but could also be helped by adding colloidal clays like bentonite or colloidal kaolin, or by including gums such as methyl cellulose or sodium alginate. Writers differed on the usefulness of each approach and some calamine lotions used both techniques.
[Calamine lotion] is the most popular of all preparations of calamine. A good formula is:
% Calamine 5-10 Zinc oxide 5 Glycerin 3 Rose water to 100 After mixing, the product should be brushed through a fine sieve, not coarser than 120 mesh. Non suspending agent in the form of gum, etc. should be added; the lotion will separate on standing but shakes up very readily.
An improved calamine lotion which remains in more or less permanent suspension can be prepared as follows:
% Calamine 15 Bentonite 2.5 Rose water to 100 (Rae, 1946, p. 200)
As calamine lotion is generally applied in small spots on the skin – as, for example, when treating poison ivy – its cosmetic effect was often ignored, with the skin becoming dotted with small pinkish-whitish dots after application. However, it was also included in complexion clays, in cosmetics for clearing up minor skin blemishes, e.g., Cyclax Special Lotion, and in some early sunburn lotions.

Above: 1946 Cyclax Special Lotion being applied with a brush.
Some cosmetic chemists thought calamine lotion could be used as a powder base if appropriately coloured. Bolton (1935) suggests that the most suitable colour is dark rachel with a reddish tinge: “a light rachel is to be avoided as it leaves the skin with a ‘white-washed’ effect, while too brown a shade will make the suspension itself appear dull and dirty” (Bolton. 1935, p. 153) and he included a suitable recipe:
Zinc carbonate 10.0 Zinc oxide 10.0 Ochre, dark shade 2.5 Armenia Bole 0.5 Iron sesquioxide 0.2 Glycerin 5.0 Rose water to 100 (Bolton, 1935, p. 153)
Wet White
Liquid powders were also used in theatrical make-up, the most common being wet white. As it was water-based it could be applied more thinly than loose powder and washed off with soap and water. It was also less likely than greasepaint to come off when body contact was made, making it an ideal body make-up for artists like dancers and members of the chorus.

Above: An actress at the end of the Edwardian period with what looks like wet white covering her arms, upper body and legs. The chorus girls also appear to be wearing wet white but not on their legs. In order to get an even finish the product was applied with a sponge, cloth or brush.
Wet white could be compounded fairly easily by mixing zinc oxide with glycerine and rose-water and anyone who wanted to make it could mix some up relatively easily. This was a common practice with chorus girls as it was cheaper that buying it ready-made and it also enabled them to mix it to a strength that best suited them.
Unlike calamine lotion – which for medical reasons was made only from zinc salts – wet whites often included other white pigments such as assorted bismuth compounds, barium sulphate, magnesium sulphate and sodium phosphate. When it became widely available, titanium dioxide was generally preferred as it was whiter and, being more opaque, had a better covering power. Other variations in the basic formula included adding other pigments, such as yellow ochre, to adjust the colour and some sort of alcohol, such as methylated spirits, to speed up the drying time. Other ingredients could be also included to help improve and maintain the suspension. Poucher (1935) mentions the use of starch but, as with calamine lotions, colloidal clays and gums were also employed.
Wet White is a theatrical requisite in great demand by the ladies who use it to whiten the neck and arms. It is known under several names, such as, Blanche de Perle [sic.], French white, etc. The basic ingredients are zinc oxide and the oxychloride, subnitrate or carbonate of bismuth. The liquid consists of a mixture of glycerine and water in varying proportions. The former is employed up to 30 per cent and the latter is often replaced by triple rose or other fragrant water. The addition of starch is desirable, as it helps to suspend the heavier ingredients. The powders are well mixed in a large mortar and the glycerine added. The water is gradually poured in to make the desired volume and the whole passed through muslin to remove grit, etc.
No. 1661 Bismuth subnitrate. 50 Starch. 50 Zinc oxide. 100 Glycerine. 150 Rose water. 650 1000
No. 1662 Bismuth carbonate. 75 Zinc oxide. 125 Glycerine. 300 Alcohol. 100 Orange flower water. 400 1000 The above products, after shaking, are well rubbed into the skin and allowed to dry. The result is an alabaster-like epidermis into which much life can be infused by the liberal application of a tinted powder. The pores become blocked, and it is therefore necessary to thoroughly cleanse the skin with soap and water after use.
(Poucher, 1935, pp. 542-543)
Like other forms of stage and screen make-up, wet white crossed over from theatrical into domestic use. Some of this transfer would have been indirect, such as dancers using the product off-stage, but a number of theatrical suppliers advertised wet white directly to the general public using its links to the theatre or motion pictures as a selling point.
Liquid Pearl
Also known by names like ‘lily white’ or ‘blanc de perle’, these liquid powders were popular in the nineteenth century where they were used to restore a smooth, white complexion to skin marred by sunburn, freckles or other skin blemishes. Although they could be used on the face, they were more commonly applied to the neck, décolletage, back and arms, primarily at night to even the tone of skin exposed by short sleeved or low cut evening dresses.
A powder for which there is much demand is that made by mixing one of these strongly white ingredients with water and thus making a so-called “liquid powder.” Although useful for the neck and arms when unsightly blotches or redness prevent making a good appearance, the application upon the face should be made most sparingly lest the skin be made dry and scaly.
Liquid Cosmetic Oxide of zinc 1 ℥ Barium sulphate 1 ℥ Glycerine 1 ℥ Alcohol 1 ℥ Water 6 ℥ Cologne ½ ℥ Mix thoroughly and strain through cotton or fine bolting cloth. Keep in a tightly corked bottle and apply with a sponge when required.
Equal in importance to the proper application of the powder is the manner of removing it, and if the skin is to be kept in a thoroughly fine condition the powder must positively be removed by a thorough cleansing each night. The most harmless substances may cause a great deal of trouble unless this precaution is observed. When traveling, a cleansing creme will remove the dust and the powder quite as effectively as soap and water. On other occasions the ordinary means may be employed. The respect with which this cosmetic is held by some people may well be estimated by the very general use it enjoys.(Lloyd, 1904, pp. 104-105)
Commercial examples available in the late nineteenth and/or early twentieth century include: Champlain’s Liquid Pearl, Stoddart’s Peerless Liquid, Hagan’s Magnolia Balm, Laird’s Bloom of Youth or Liquid Pearl, Pomeroy’s Liquid Powder, and Madame Yale’s Excelsior of Beauty.
As with calamine lotion and wet white, liquid pearls were made by mixing powder in plain water, rose-water or orange-flower water to which glycerine had been added. Some early forms contained lead white (lead carbonate) but by the turn of the century most of the pigments used were innocuous, like those in loose face powders; e.g., zinc white (zinc oxide), pearl white (bismuth oxychloride), chalk (calcium carbonate) and titanium dioxide.
The advantages that liquid pearls had over loose powders were the same as those for theatrical wet white, to which they were closely related: they could be applied more thinly, so were potentially less noticeable; and were more resilient to wear and tear, making them a useful to women going out for an evening of dining and dancing.
Like wet whites, many of these early liquid pearls required shaking before use to ensure that the powder was evenly suspended and they were also applied with a sponge, cloth or brush to reduce streaking.
Liquid substitutes for powders commend themselves strongly to many persons, and under some conditions have advantages. For instance, they last longer, and at night give a satisfactory finish to the complexion. But one fact always to be remembered when using them is that they must be put on with extreme care and evenness, otherwise there will be patches of white which spoil one’s looks.
The simplest liquid is composed of one ounce of pure oxide of zinc, a dram of glycerine, four ounces of rosewater and fifteen drops of essence of rose. To mix, the glycerine is slowly poured over the zinc, stirring all the time to keep a smooth paste. The rosewater follows, added in the same manner. The essence goes last. When bottled there will be a white sediment at the bottom, and the preparation must always be shaken before any is put on the face. In applying a piece of muslin or linen should be used.(Mixter, 1910, p. 118)
Being liquids, some manufacturers also added skin lighteners, such as corrosive sublimate (bichloride of mercury) to help lighten the skin by ‘bleaching’ it over time.
See also: Bleach Creams and Mercolized Wax
Analgesics
Two water-soluble analgesics – phenazone and acetanilide – were also used in liquid powders.
Phenazone: Known by the trade name Antipyrin (also antipyrine), phenazone was discovered by the German chemist Ludwick Knorr [1859-1921] in 1883. The drug had antipyretic (fever reduction), analgesic (pain numbing) and hemostatic (blood flow reduction) properties. Large qualities of it were manufactured as patients needed to take a lot of it each day for it to be effective (Hinx & Brune, 2004, p. 2393).
When dissolved in water, antipyrin was colourless but when applied to the skin it formed a fine, white powder that had a very attractive peach bloom quality after the water evapourated. It also blanched the skin somewhat, so was useful in reducing redness. Like wet white, it could also be made with a solution of alcohol in water which would speed up the rate of evapouration.
Phenazone seem to have been quite commonly used in liquid powders, although perhaps its use was more widespread in Europe than elsewhere. Winter refers to it as Eau Mystérieuse suggesting that the idea of using it as a cosmetic stemmed from France. He gives two formulations for the product, here translated:
Antipyrin 10 g Rose water 10 g Orange flower water 20 g Water 55 g Glycerol 5 g Ionone 11 Tr. (Winter, 1927, p. 636)
Antipyrin 100 g Glycerol 100 g Rose water 400 g (Winter, 1927, p. 636)
Technically, Eau Mystérieuse is a solution, not a suspension, so it is not a liquid powder; however liquid powders were also made that included Antipyrin. For example, Henri Fouquet, the Parisian perfumier, suggested using it in an evening lotion for the neck, arms and shoulders made to a formula similar to the one given below:
Distilled water 200 c.c. Rose water 450 c.c. Glycerin 200 c.c. Antipyrin 200 g. Colloidal kaolin, whitest 400 g. In such a formula the antipyrin may be dissolved in approximately 600 c.c. of the mixed water and rose water. Shake the kaolin thoroughly with the remainder of the mixed solution after first making a paste with it. Mix the two liquids, then add the glycerin. The proportions of glycerin may be increased if desired. Also magnesium stearate, talc, titanium dioxide, magnesium carbonate etc. may be incorporated.
(Vallance, 1940b, p. 168)
No matter how attractive it looked on the skin, antipyrin was a water-soluble drug and was therefore dangerous to use. That, and the fact that it was incompatible with a wide range of substances, meant that it disappeared from cosmetics sometime before 1950.
Acetanilide: Discovered by two young doctors, Arnold Chan and Paul Hepp in 1886, acetanilide was known by the trade name Antifebrin because was a febrifuge (reduces fever) as well as an analgesic. Like phenazone, it was water-soluble and formed a fine white powder on the skin after the water had evapourated. Despite the fact that it was shown to be highly toxic – with symptoms including slow breathing, feeble pulse, collapse, coma and death – this apparently did not stop it from being used in some cosmetics in a fashion similar to phenazone. Warnings against using it were still being made well into the twentieth century.
No use whatsoever must be made of those clear solutions which, applied to the skin and allowed to evaporate, leave a white light powder, for they are extremely dangerous, particularly because of their daily use and their content of acetanilide.
(Gattefossé, 1949, p. 128)
Developments in formulation
Between 1900 and the outbreak of the Second World War, cosmetic chemists attempted to formulate the ideal liquid make-up; one that was fast drying, resisted settling, poured easily, felt smooth, was not sticky or greasy, resisted rubbing off, and made in a good blend of colours.
Improving the suspension: The settling issue was never completely solved but it was ameliorated to a considerable extent. As with wet white and calamine lotions the solution to this problem generally involved getting the powdered pigments as fine as possible, altering the proportions of the mixture, adding suspending agents such as colloidal clays, and/or increasing the viscosity of the mixture by including mucilages or gums.
On suspending the zinc-white in water in the manufacture of liquid face powder, it soon settles to the bottom of the bottle, and must be vigorously shaken before use. But if the amount of zinc white is so chosen that it forms just a little more than half the amount of water used, and if a very fine kind of zinc-white of low specific gravity is taken, then the zinc-white remains suspended in the water and settles only slightly, without forming a sediment. The emulsion under all circumstances therefore remains milk white. This can also be accomplished with a somewhat heavier and not so fine zinc-white, by adding to the water traces of gelatin. In the later case some salicylic acid dissolved in alcohol must not be omitted, else there is a danger of the liquid becoming mouldy. The addition of water has the advantage, in that the moist zinc-white can be more evenly distributed on the skin than when the dry preparation is used. Small quantities of chemically pure glycerin are made to these liquid preparations, so as to counteract any effects that the moisture may have on the skin. Such liquid powders can also be easily wiped off from the skin, but resist the action of strong air currents. They can be so deeply rubbed into the skin, without any ill effect on it, as to be absolutely invisible to the layman. Often it suffices to add a small quantity of glycerin, so that good zinc-white will remain in suspension even without the addition of gelatin, i.e., they will not settle. These liquid powders are preferable to others, because the gelatin, even though added in very small quantities, still tends to clog up the pores to a certain extent, which is undesirable in all cases.
(Mann, 1914, p. 294)
As the century progressed, the ability of cosmetic chemists to micronise powders improved dramatically and by the 1940s they were able to make powders of a fineness that could have only been imagined in previous decades. This not only decreased their tendency to settle but also made the powder feel a lot smoother on the skin.
See also: The Bite Test
As mentioned earlier, cosmetic chemists could also improve the suspension by adding suspending agents such as corn or rice starch or, even better, include a colloidal clay, such as osmo-kaolin, bentonite or wilkinite, to slow the rate of settling. In the 1940s, deNavarre carried out some experiments using a range of suspending clays and found bentonite to be the most effective. However, being an American clay it was not always available elsewhere and osmo-kaolin was frequently used instead.

Above: “Stability of water suspensions of pigments. No. 1 zinc oxide 10%, No 2. colloidal kaolin 10%, No. 3 10% talc (note heavy deposit on bottom), No. 4 zinc oxide 10% plus 3% bentonite. Pictures taken 4 hours after shaking” (deNavarre, 1941, p. 636). The most useful suspending agent according to deNavarre is bentonite. It forms a good suspension when shaken and leaves a clear supernatant when the powder settles.
Some manufacturers also followed the earlier practice of including substances that increased the viscosity of the mixture, such as gums and mucilages, but, as deNavarre pointed out, these were not without their problems.
Liquid powder is probably the most useful preparation for make-up of arms and shoulders. Women who “go out for dinner” a lot, always keep a bottle on hand.
Liquid powders are also suggested as a “powder base” prior to application of ordinary face powder. Such a preparation can hardly serve as a powder base, other than increasing the covering power of the face powder to be used.
Formularies recommend the use of gum mucilages to aid in maintaining a suspension. Experiments show that there is quite a bit of incompatibility between most gum mucilages (especially quince) and pigments used. Acacia may be used in some instances, but as little as 1% causes the pigments to form a solid rock-like mass at the bottom of the bottle.(deNavarre, 1941, p. 636)

Above: 1936 Liquid powders from Claire Waters, Elizabeth Arden and Richard Hudnut. These were probably shaken before being photographed.
Colour: Early liquid powders were generally only lightly coloured in white, pink or rachel tints. However, as women began to participate in outdoor sports and take up suntanning, liquid powders were needed to cover strap marks and other pigmentation unevenness caused by sunbathing, so that the transition from summer holidays to city living would be smoother. Some liquid powders of the 1920s and 1930s therefore included shades that could be used on darker skin tones. For example, Dorothy Gray’s Finishing Lotion, introduced in 1929, came in seven shades: Blonde (a delicate flesh pink), Natural, Aureate (with a hint of peach), Rachel, Tawny (a warm golden tan), Orchid (for evening use) and Sunburn (for those who have acquired a tan). Some manufacturers of the time went even further and promoted their darkly coloured, liquid powder make-up as a liquid tan, e.g., Coty’s Cotytan.
This is not to say that all liquid powders made in the 1950s were highly coloured. Elizabeth Arden’s Ardena Lille de France for sensitive skins, and Ardena Lille Lotion for those with oily or blemished skin, came in lighter shades. Both products needed to be shaken before use. Arden turned this weakness to her advantage by suggesting women could adjust the covering power of each by pouring off some of the supernatant before shaking. Although many women found this useful, Ardena Lille Lotion was a bit of an antique by the 1950s, having been around since before the First World War when it was known as Venetian Lille Lotion.

Above: Assorted make-up and foundations from Elizabeth Arden. Ardena Lille de France and Ardena Lille Lotion are liquid powders. Note that the powder has settled in each bottle leaving a clear supernatant. They came in a large bottle because they were used to cover large areas of the body such as the arms and shoulders.
See also: Elizabeth Arden
Other qualities: Cosmetic chemists could also adjust particular features of their liquid powders so as, for example, to make it faster drying or improve its smoothness.
Formulation of Liquid Powders
1. Standard
type2. With
chalk3. Quick
drying4. Extra
smoothStarch, rice or corn … … 5 5 Colloidal kaolin 10 10 10 15 Precipitated chalk … 5 5 … Zinc oxide 16 10 … … Titanium dioxide … … 5 5 Glycerin 8 5 10 7 Alcohol 10 7 9 10 Perfume compound 1 1 1 1 Tincture Benzoin … … 5 … Quince seed mucilage … … … 3 Distilled water 105 112 100 104 (Lee-Charlton, 1936, p. 62)
Leg make-up
Leg make-up had an enormous influence on the evolution of liquid face powders – and make-up bases or foundations in general. Coloured body make-up was available well before the war, and was used by some to replace stockings at the seaside or in other places where wearing them would be unsuitable. However, it was not until stockings became scarce during the Second World War that most women became desperate enough to try it.
See also: Cosmetic Stockings
Leg make-up was also used in the theatre well before 1939. On the Continent, dancers had applied a coloured wet white known as ‘bas de soie’ (silk stockings) as a stocking replacement. Jannaway reports Cerbelaud citing the following formula for this type of cosmetic. Although the formulation is for a cream, it could be easily modified to produce a lotion.
Grams. Titanium dioxide 50.0 Zinc oxide 200.0 Venice talc 250.0 Colloidal kaolin 200.0 Cobalt blue 0.5 Rose lake 2.0 Distilled rose water to 225.0 Glycerin 25.0 Oil of lavender 2.0 Coumarin 0.5 Bergamant oil 1.0 Rhodinol 0.5 Oak moss 0.05 Opoponax, synthetic 1.0 The powders are intimately mixed and made into a base with the glycerin and water. The perfumes are then added, with constant mixing, and the product is filled into jars.
(Jannaway, 1939, p. 130)
The difficulties in making a good liquid leg make-up were the same as those for earlier liquid powders – it had to go on with out streaking, cover well, and not be easily rubbed off – with the additional requirement that it mimic the colour and texture of silk or nylon as closely as possible. Although many early products were less than satisfactory, by the end of the war, trial and error had enabled many cosmetic companies to develop highly serviceable leg cosmetics.
When the war ceased and the stocking shortage ended, the demand for leg cosmetics declined. However, many companies used what they had learned in the formulation of cosmetic stockings to develop new liquid and cream face powders, commonly referred to as make-up bases or foundations.
Make-up bases and foundations
The formulation of liquid make-up bases went in a number of directions after the war. An early liquid powder foundation developed by the House of Westmore called Overglo enjoyed some popularity for a time. It was an oil suspension covered by a British patent (GB577040: 1946) which included a suggested formulation:
Sesame oil 64 parts Zinc oxide 11 parts Titanium dioxide 16 parts Oxy-cholesterol 2 parts Para hydroxy benzoic acid Q.S. Glycerol tri-stearate 1 parts Perfume and colouring matter 6 parts (British Patent No. GB577040: 1946)
Another pioneering patent was granted to Carl Weeks of the Armand Company (US2373933: 1945). It used a combination of bentonite and methyl cellulose as suspending agents in a liquid containing sorbitol, propylene glycol or glycerin. The cosmetic adhered well to the skin and when the water evapourated the remaining film looked like a face powder.
Although some liquid powders, such as Overglo, were made without water, most liquid make-up powders developed after the war were emulsions made from surfactants, suspending agents, wetting or spreading agents, pigments and fillers. As such they were dramatically different from the wet whites, calamine lotions and liquid pearls used decades earlier.
An early example of this new type of emulsion-based liquid powder was Liquid Beauty first sold in 1946; an oil-in-water emulsion developed by the renowned cosmetic chemist Maison G. deNavarre for the door-to-door cosmetic company Beauty Counselor. Although it was a cream rather than a liquid it pointed in the direction that most liquid powders of post-war period would go, namely that they would be emulsions. Liquid powders of this type included Westmore’s Tru-Glo, Revlon’s Touch and Glow (1950), Helena Rubinstein’s Silk-Tone, Richard Hudnut’s Flatter-Glo (1953), Coty’s Instant Beauty (1952), Max Factor’s Hi-Fi (1955), and Dorothy Gray’s Sheer Velvet Film.
Most of these liquid foundations were highly pigmented and only a small amount was needed to cover the face. They were usually applied using the fingers rather than a cloth, sponge or puff, which gave then an advantage over make-up like Pancake that had to be applied with a wet sponge. It also eliminated the wastage experienced with some earlier liquid powders like Elizabeth Arden’s Adrena Lille Lotion where a lot of the lotion was discarded with the used cotton wool pad. Their concentrated formulation also meant that they were generally sold in small bottles making them convenient to carry around in a purse.
Some of these post-war liquid powders were supposed to be followed by a covering of loose powder, which might be transparent if the liquid powder provided all the colour needed, or shaded with a complementary colour if not. However, before long, stand-alone formulations that did not require a follow-up powder became widely available, and these have been in continuous production to the present day.
Matte Liquid Make-Up
Parts by weight Stearic acid XXX 3.2 Propylene glycol monostearate (pure) 2.00 Lanolin alcohols 1.80 Sorbitan sesquiolate 0.50 Polyparaben 0.10 Isopropyl myristate 6.50 Triethanolamine 1.60 Methylparaben 1.10 Gum 0.25 Polyethylene glycol 600 15.00 Pyrogenic silica 3.00 Kaolin 0.75 Titanium dioxide 8.50 Iron oxides 1.50 Water 55.00 Perfume q.s. (Schlossman & Feldman, 1975, p. 753)
Unfortunately, many still require shaking before they are applied.
First Posted: 1st September 2014
Last Update: 23rd July 2020
Sources
Bolton, F. J. (1935). Calamine in pharmacy and cosmetics. The Industrial Chemist and Pharmaceutical and Cosmetic Supplement. December, 153-154.
deNavarre, M. G. (1941). The chemistry and manufacture of cosmetics. Boston: D. Van Nostrand Company.
Forget-Me-Not. (1893). London: Periodical Publishing Co. Ltd.
Gattefossé, R. M., & Jonquières, H. (1949). Technique of beauty products. (A.R.I.C., Trans.). London: Leonard Hill.
Jannaway, S. P. (1939). Theatrical make-up. The Perfumery and Essential Oil Record. April, 128-132.
Lee-Charlton, H. (1936). Liquid powders and wet whites. The American Perfumer and Essential Oil Review. November, 61-62.
Lloyd, E. (1904). The skin. Its care and treatment (2nd ed.). Chicago: McIntosh Battery and Optical Company.
Mann, H. (1914). Zinc oxid in cosmetics. The American Perfumer. VIII(12), February, 294-295.
Mixter, M. (1910). Health and beauty hints. New York: Cupples & Leon Company.
Rae, J. (1946.) Calamine. Manufacturing Chemist and Manufacturing Perfumer XVII(5), May, 199-200.
Schlossman, M. L., & Feldman, A. J. (1975). Fluid foundation and blush make-up. In M. G. deNavarre, The chemistry and manufacture of cosmetics (2nd ed., Vol. IV). Orlando: Continental Press.
Vallance, J. M. (1940a). Liquid make-up. Manufacturing Chemist. May, 145-147.
Vallance, J. M. (1940b). 4. Some make-up specialities. Manufacturing Chemist. June, 168-169, 172.
Winter, F. (1927). Handbuch der gesamten parfumerie und kosmetik. Vienna: Springer-Verlag.

1873 Hagan’s Magnolia Balm. Originally developed in 1850 to an unknown formulation, by the 1880s it was made from zinc oxide and water. By 1910, it was being sold in white, pink and red shades.

1886 Champlin’s Liquid Pearl.

1889 H. R. Hale Royal Pearl.
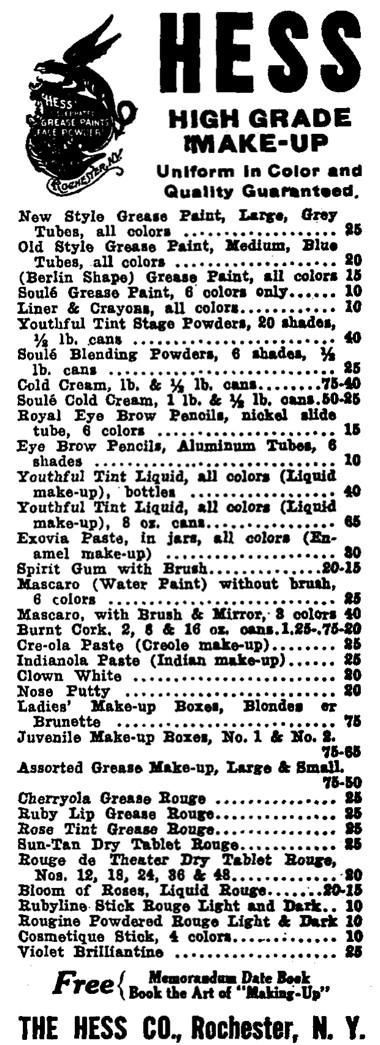
1913 Hess stage make-up including Youthful Tint liquid make-up produced in a range of shades.

1913 Wakelee’s Camelline.

1914 Cross Theatrical Liquid Make-up.

Richard Hudnut Orchid Beauty Cream (Smithsonian). Introduced sometime before 1915 it was described as: “A harmless, instantaneous beautifier for the face, neck and arms suited to the requirements of society women who do not want exaggerated effects. It suffices to apply the cream with a small sponge and when dry, to rub the skin gently with a soft cloth or piece of chamois leather.”

1929 Article on applying a fake tan from the July edition of the Pictorial Review.

1929 Coty Cotytan. It came in two shades, blonde and brunette. It was applied to the face using a small pad of wet cotton but was smoothed over the rest of the body with the palm of the hand.

1929 Kathleen Mary Quinlan artificial tan.
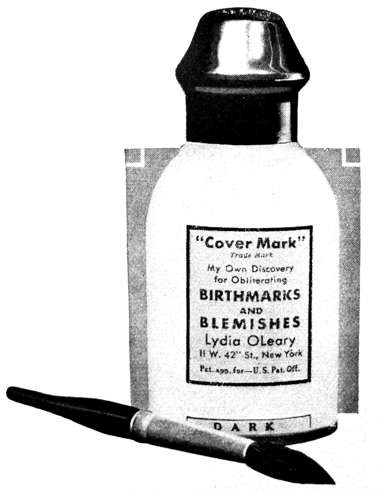
1931 Lydia O’Leary Covermark Lotion. This liquid powder was used as a concealer to hide birth marks and other skin blemishes. O’Leary found that it did not cover the skin as well as expected and soon switched from a liquid powder to a powder cream.

1935 “Old Drury” Wet White on sale in Singapore.

1936 Cyclax Sunburn Lotion and other preparations. The bottles from the back to the front are: Bracine Skin Tonic, Cleansing Lotion, Astringent Complexion Milk, Sunburn Lotion and Special Lotion. The Sunburn Lotion has separated into two parts and would need to be shaken to suspend the powder before it was applied.

1940 Crookes’ Lacto-Calamine. A perfumed lotion containing kaolin, zinc oxide, zinc carbonate, castor oil and assorted emulsifiers, colours and preservatives. Highlighting the protecting, soothing and healing properties of calamine the product was also advertised as an antidote to sunburn along with suggestions that it could be used as a powder base.

1940 Miner’s Liquid Make-up. The formulation of stocking substitutes during the war stimulated the development of new forms of liquid make-up.

1943 Elizabeth Arden Velva Leg Film. Arden was making this cosmetic well before the Second World War broke out.

1944 Vida-Ray VidaFilm liquid cake make-up.

1945 Westmore Overglo Liquid Cream Foundation Make-up.

1945 Barbara Gould Liquid Velvet.

1952 Westmore Tru-Glo Liquid Make-up.

1952 Coty Instant Beauty.

1954 Beauty Counselor, the makers of Liquid Beauty.

1955 Cyclax Special Lotion.

1955 Richard Hudnut Flatter-Glo Liquid Make-up.

1955 Max Factor Hi-Fi Fluid Make-up.

1957 Dorothy Gray Sheer Velvet Film.

1958 Starlet Beauti-Film Liquid Make-up.

1960 Helena Rubinstein Silk-Tone Liquid Make-up.

1961 Revlon Touch and Glow.

1964 Harriet Hubbard Ayer Liquid Powder.

1964 Tussy Finishing Touch. Post-war consumers were offered a much wider range of make-up formulations than had been available before the war and cosmetic companies now gave consumers a choice between powders, creams and liquids.

1966 Elizabeth Arden Sheer Cover liquid leg make-up. Arden was one of the first of the majors to get into leg make-up and she stuck with it.
