Ban
In 1931, Bristol-Myers acquired the Mum Manufacturing Company of Philadephia, the makers of Mum deodorant. In 1948, the company resolved to add an antiperspirant to the Mum range and by 1951 the chemists in its Product Division had created and tested a suitable antiperspirant lotion.

Above: A chemist from the Products Division of Bristol-Myers (Bristol-Myers Annual Report, 1955).
Bristol-Myers decided to market this new product in a dispenser modelled on the ball-point pen, an approach that had not previously been tried in this market. This idea may have come from Helen Leah Diserens [1919-2008] who was working for Bristol-Myers at the time. A roll-on mechanism for the deodorant/antiperspirant was then developed and patented (US2,749,566, 1956).
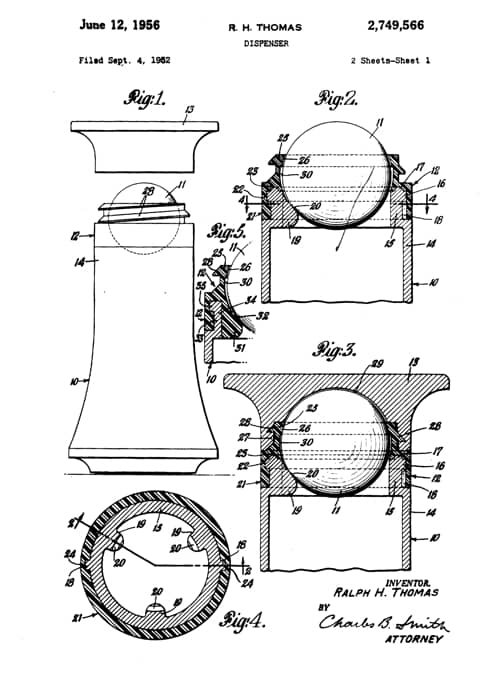
Above: Drawings from the 1956 patent for the roll-on dispenser assigned to Bristol-Myers.
In 1952, the new antiperspirant lotion in its ball-point package went on sale in the American market in Philadelphia, Baltimore, Washington, Cleveland, Dayton and Columbus. Called Mum Rollette, the early reactions were good but unfortunately the applicator seized up with use, generating a string of complaints. This caused Bristol-Myers to remove the product from sale and to do further research.
Development issues
Bristol-Myers was not the only company to get into difficulties when developing a cosmetic dispensed as a roll-on. Richard Hudnut also experienced problems when it released Lip Quick, a roll-on lipstick, in 1959.
See also: Richard Hudnut
Examination of the Rollette dispensers indicated that the active ingredients in the antiperspirant had affected the plastics used in the marble and the neck-ring that held it in place. Each plastic had expanded differently over time causing the marble to stick. During the redevelopment of the roll-on dispenser Bristol-Myers tested hundreds of different combinations of glass, plastics and metals before settling on a glass bottle, a neck-ring of polyethylene, and a marble and cap both of hard polystyrene.

Above: Examining material combinations of the four components of the container – bottle, cap, marble and neck-ring (Bristol-Myers Annual Report, 1955). Some of the proposed bottles look similar to those drawn in the patent submitted in 1952, granted in 1956.
A second issue was the marble itself. It had to be an almost perfect sphere to ensure that the antiperspirant lotion flowed evenly and the marble would not jam. A supplier was eventually found who could create a suitable spheres by grinding them from slabs of hard polystyrene.

Above: Testing marble samples to ensure they were near-perfect spheres (Bristol-Myers Annual Report, 1955).
Unlike most roll-ons sold today, the cap used on the early Ban antiperspirants was transparent. This enabled potential buyers to see the roll-on mechanism which was itself a major selling point.
Learning from their earlier failure with Rollette, Bristol-Myers also put the new container through extensive testing to ensure it would withstand the expected wear and tear.
Delivery
The product was called Ban rather than Rollette – possibly to distinguish it from the earlier failure – and it was given a black and gold label designed to appeal to men as well as women. Full-scale production of Ban began in the United States in December, 1954, with initial shipments starting in February, 1955.

Above: Packaging bottles of Ban antiperspirant for delivery across the United States (Bristol-Myers Annual Report, 1955).
Then, in March, 1955, Bristol-Myers rolled out a national advertising campaign combining print, radio and television.

Above: 1955 Arthur Godfrey [1903-1983] promoting Ban on his daytime television and radio show.
Sales exceeded expectations and Ban became the third highest-selling deodorant in the United States within a year, even though its price point was higher than its rivals. One contributing factor to its success was that demonstrating the roll-on mechanism was ideal for television.
Following its success in America, Bristol-Myers began selling the roll-on antiperspirant overseas but there it was marketed as Mum Rollette. The Mum label was used as it had high brand recognition and consumers had not been exposed to the earlier Mum Rollette failure which had only been sold in the United States.
Later developments
The roll-on was widely copied and remains a common dispenser for deodorants and antiperspirants to this day. Despite the ‘revolutionary’ nature of the roll-on, Bristol-Myers developed other forms of Ban using different delivery systems to counter its competitors. The first of these was Ban Cream Deodorant (1963) which was applied using the fingers. Later Ban products included Ban Spray Deodorant (1965), Dry Ban Aerosol Antiperspirant (1968), Ultra Ban 5000 Aerosol Antiperspirant (1971), and Ultra Ban Super Dry Aerosol Deodorant (1974).
See also: Deodorants and Antiperspirants
First posted: 7th March 2017
Last Update: 5th July 2022
Sources
Bristol-Myers, Inc. Annual Reports 1950-1975.
How Ban rose to number 3 deodorant in one year. (1956). Sponsor, April 16, 40-41, 70, 72, 75.
Laden, K. L. (Ed.). (1999). Antiperspirants and deodorants (2nd ed.). New York: Marcel Dekker, Inc.
Van Nostrand. R. K. (1956). The ‘Ban’ development story … product, people, planning. Drug and Cosmetic Industry, 79(6), 777, 779, 781, 834-835.

Ban Lotion Deodorant package and dispenser. The dispenser had a transparent cap made from hard polystyrene so that the ball-point mechanism was visible. A cut-out in the package also allowed the customer to see this in the box.

Despite the label on the front of the package and the bottle, the product was also an antiperspirant. The reverse side of the package lists this as aluminum chlorhydroxide.

1955 Ban Lotion Deodorant.

1956 Trade advertisement announcing the arrival of Mum Rollette in Britain just in time for summer.

1957 Mum Rollette (Britain).

1960 Mum Rollette for men (Italy).

Ban Roll-On Anti-Perspirant and Cream Deodorant.

1963 Ban Deodorant Cream.

1970 Ban Roll-On.
